
How many meters of 34-gauge wire (diameter = 6.304 x 10¯ 3 inches) can be produced from the copper that is in 5.88 pounds of covellite, an ore of copper that is 66% copper by mass? (Hint: treat the wire as a cylinder. Problem #21: Copper can be drawn into thin wires. The rest of the cube (45.0 − 29.8) is above the surface of the liquid. This means that 29.8 mL of the cube is submerged (this is the answer to the question), displacing 40.5 g of the liquid. We need to know what volume of the liquid weighs 40.5 g. The solution to this problem involves the concept of buoyancy.Ģ) The cube will float when 40.5 g of liquid is displaced. What volume of the cube is submerged in the liquid? Problem #20: An ice cube with a volume of 45.0 mL and a density of 0.900 g/cm 3 floats in a liquid with a density of 1.36 g/mL. Solution: Object A has a larger volume than Object B. If both objects are the same mass, what can be said about the volume of A as compared to the volume of B? Problem #19: Object A is less dense than object B. Calculate the total mass if ice has a density of 0.92 g/cm 3. Problem #18: Antarctica has an ice sheet covering 1.42 x 10 18 cm 2 and averaging 1.61 x 10 5 cm deep. Since this mass is in 1.00 cm 3, the answer is 7.25 g/cm 3
This means:Ĭopper occupies 0.06025 cm 3 and zinc occupies 0.93975 cm 3. If a penny contains 93.975% of its total volume zinc and 6.025% of its total volume copper, what is its apparent density? (density of Cu = 8.96 g/cm 3 density of Zn = 7.14 g/cm 3.)ġ) Assume the penny occupies 1.00 cm 3. Problem #17: In the United States, 'copper' pennies made since 1983 actually contain very little copper. What is the length of wire in meters? Cu density = 8.96 g/cm 3. Problem #16: 57.0 kg of copper is drawn into a wire with a diameter of 9.50 mm. Determine the density of the ball bearing in g/cm 3. Problem #15: A spherical ball bearing has a radius of 8.50 mm and a mass of 2.315 g. Problem #14: Calculate the mass of zinc in grams (density = 7.14 g/cm 3) with the same volume as 100.0 grams of aluminum (density = 2.70 g/cm 3) Problem #13: Calculate the mass of copper in grams (density = 8.96 g/cm 3) with the same volume as 100.0 grams of gold (density = 19.31 g/cm 3)ģ) Setting up the problem in dimensional analysis style:

What mass of silver will have the same volume as 15.55 grams of benzene?

Problem #12: The density of silver is 10.50 g/cm 3 and the density of benzene is 0.8786 g/cm 3. Reminders: 1 cm 3 = 1 mL and 1000 mL = 1 L. Gas density is typically measured in g/L as opposed to g/cm 3 or g/mL. Use the data to calculate the density of argon gas.
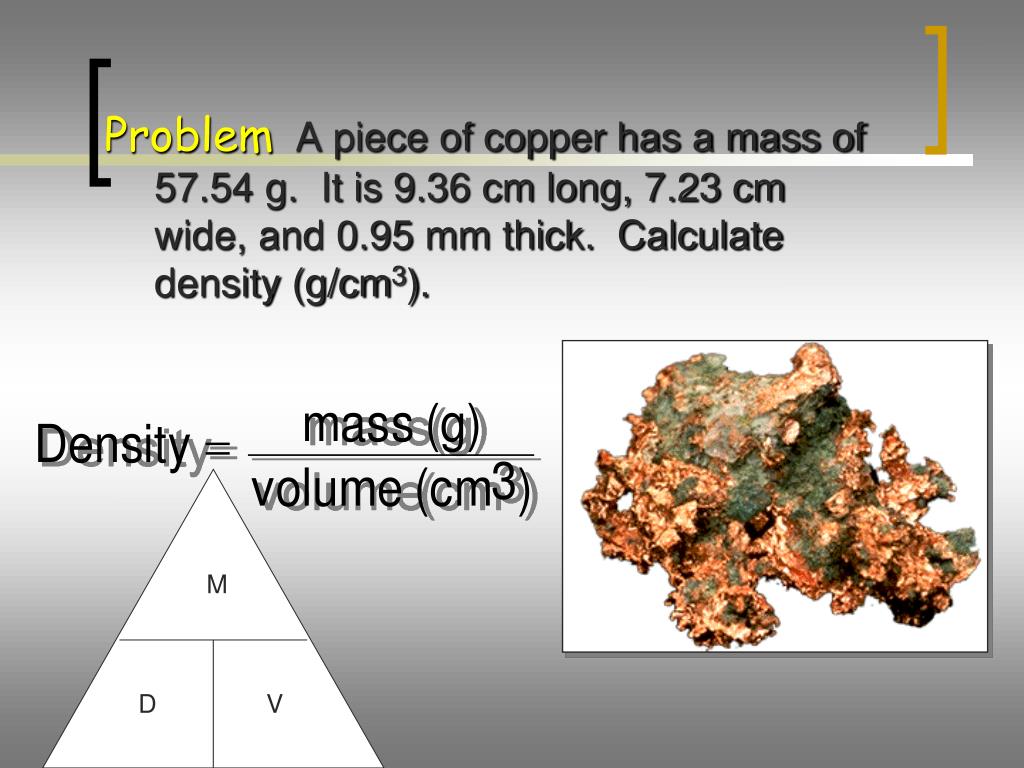
and the tube filled with argon weighs 188.870 g. Problem #11: A cylindrical glass tube of length 27.75 cm and the radius 2.00 cm is filled with argon gas. Problems 11-25 Twenty Examples Probs #1-10 Probs #26-50 All the examples & problems, no solutions Significant Figures Menu ChemTeam: Density Calculations - Problems 11-25


 0 kommentar(er)
0 kommentar(er)
